Boy with Rare Genetic Disorder Stuns Doctors Following World-First Gene Therapy
Rokna Social Desk: A three-year-old California boy with the rare and fatal Hunter syndrome has astonished doctors worldwide after becoming the first patient to receive a pioneering gene therapy in Manchester, showing rapid and remarkable recovery within months.

According to Rokna, citing BBC, a three-year-old boy has amazed doctors with his rapid progress after becoming the first patient globally with his severe condition to undergo a groundbreaking gene therapy.
Oliver Chu suffers from Hunter syndrome (MPS II), a rare inherited disorder that progressively damages the body and brain. In the most serious cases, patients often do not survive past their teenage years. The disease is sometimes described as a form of childhood dementia.
Prior to treatment, a faulty gene left Oliver unable to produce a crucial enzyme required to maintain healthy cells. In a global first, doctors in Manchester attempted to halt the disease by genetically modifying Oliver’s cells.
Prof. Simon Jones, co-leader of the trial, told the BBC: "I’ve been waiting 20 years to see a boy like Ollie doing this well, and it’s incredibly exciting."
At the center of this story is Oliver—one of five boys worldwide enrolled in the trial—and his family from California, who entrusted the Royal Manchester Children’s Hospital team with his care.
A year after starting the treatment, Oliver’s development now appears normal.
"Every time we talk about it, I feel like crying because it’s just so incredible," said his mother, Jingru.
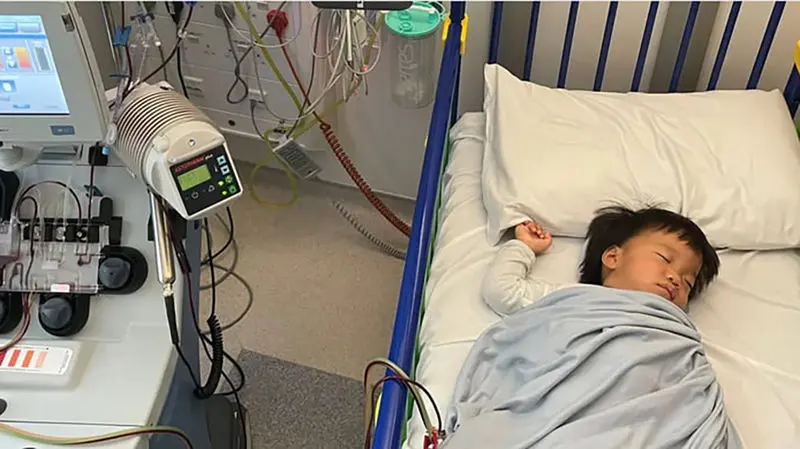
Stem Cell Collection – December 2024
Oliver and his father Ricky were first met at the clinical research facility in Manchester in December 2024. Since his diagnosis in April, his life—like that of his older brother Skyler, who also has the condition—has been dominated by hospital visits.
Skyler had experienced some delayed development in speech and coordination, initially attributed to being born during Covid. Ricky described their sons’ diagnoses as a complete shock.
"When you discover Hunter syndrome, the first thing doctors say is, 'Don’t look it up online—you’ll only find the worst cases and become very disheartened.' But, naturally, you still look it up and think, 'Is this what will happen to both my sons?'"
Symptoms typically appear around age two and can include physical changes, limb stiffness, short stature, and damage to organs such as the heart, liver, bones, and joints. Severe cases may cause significant neurological decline. Hunter syndrome is extremely rare, affecting one in 100,000 male births.
Until now, Elaprase was the only treatment, costing roughly £300,000 per patient annually, which slows physical effects but does not cross the blood-brain barrier to help with cognitive symptoms.
Today, Oliver’s stem cells were removed as the first crucial step in the experimental therapy. Dr. Claire Horgan explained: "His blood is processed through a machine to collect stem cells, which are then sent to a lab for modification before being reintroduced."
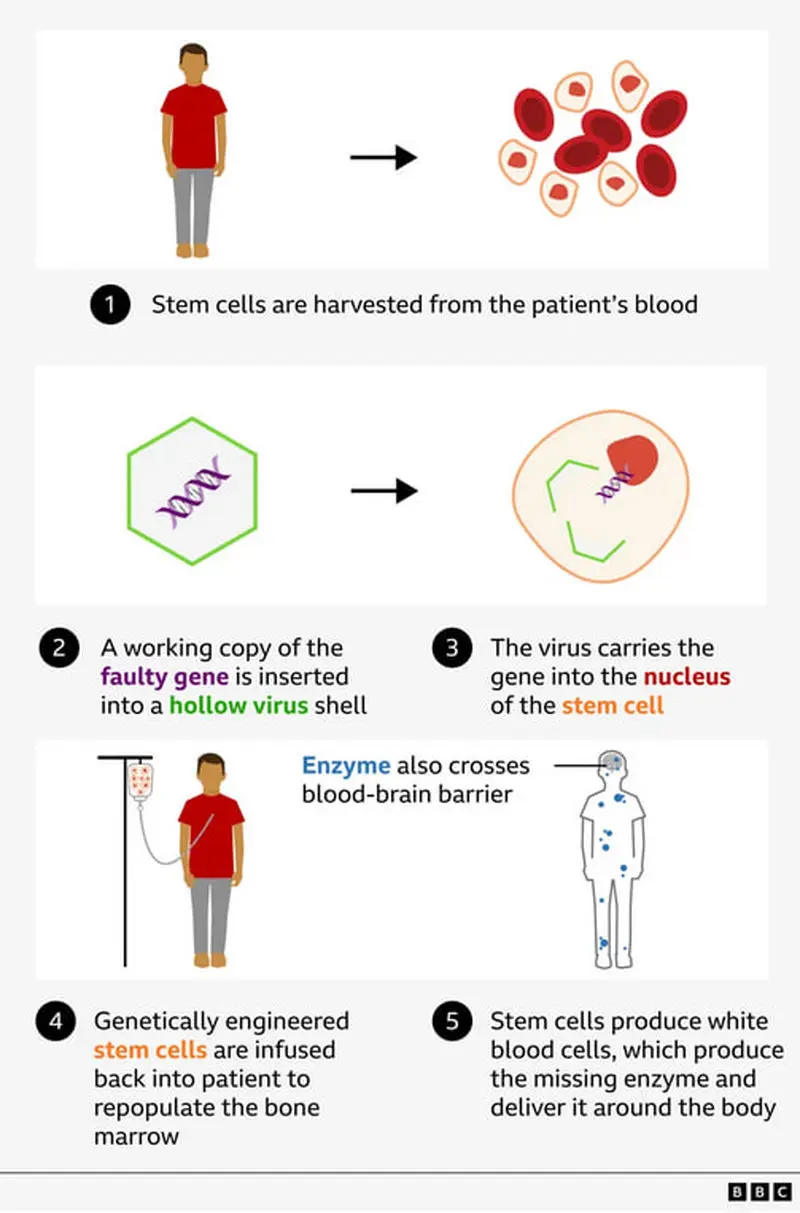
Gene Therapy Process
The cells were carefully transported to Great Ormond Street Hospital in London, where the missing IDS gene was inserted into a hollow virus shell incapable of causing disease. This method, used in other therapies like MLD, allows the modified cells to repopulate Oliver’s bone marrow and produce white blood cells that create the missing enzyme, which can now reach the brain more effectively.
Infusion Day – February 2025
Back in Manchester, Oliver received the infusion of 125 million gene-modified stem cells in two sessions, all while watching cartoons, unaware of the potential life-changing impact. Within days, Oliver and his mother returned to California, leaving doctors and family waiting to see results.

Early Signs of Recovery – May 2025
By May, tests confirmed progress. Oliver showed increased mobility, improved speech, and cognitive engagement. He no longer required weekly enzyme infusions. His mother described his transformation: "He’s so different, talking constantly and interacting with other children."
Skyler, undergoing enzyme infusion therapy but not the gene therapy, remained affectionate and protective of Oliver. Ricky expressed hope that Skyler might also benefit from the gene therapy in the future.

Ongoing Monitoring and Future Prospects
Oliver is now producing the enzyme naturally and continues to thrive nine months post-treatment. Prof. Jones noted: "Before the transplant, Ollie produced no enzyme. Now he’s producing hundreds of times the normal amount. His development is progressing rapidly."
The trial includes five boys from the US, Europe, and Australia. All participants will be monitored for at least two years. If successful, Manchester University plans to partner with a biotech firm to license the therapy. Similar trials are underway for other MPS disorders.
Ricky and Jingru expressed gratitude to the Manchester team: "We are astonished by his progress. His life is no longer dominated by hospital visits. His development has accelerated exponentially."
The trial, led by Prof. Brian Bigger, almost did not happen due to funding challenges, but British charity LifeArc stepped in with £2.5 million, allowing the first-in-human treatment to proceed.
Ricky concluded: "I would go to any lengths to ensure my children have a better future."
Send Comments