Razi Covo-Pars Vaccine to be available in mid summer
Rokna: The President of Iran University of Medical Sciences in Tehran has expressed hope that the Iranian-made Razi Covo-Pars Covid-19 Vaccine will be available to use by mid-summer.
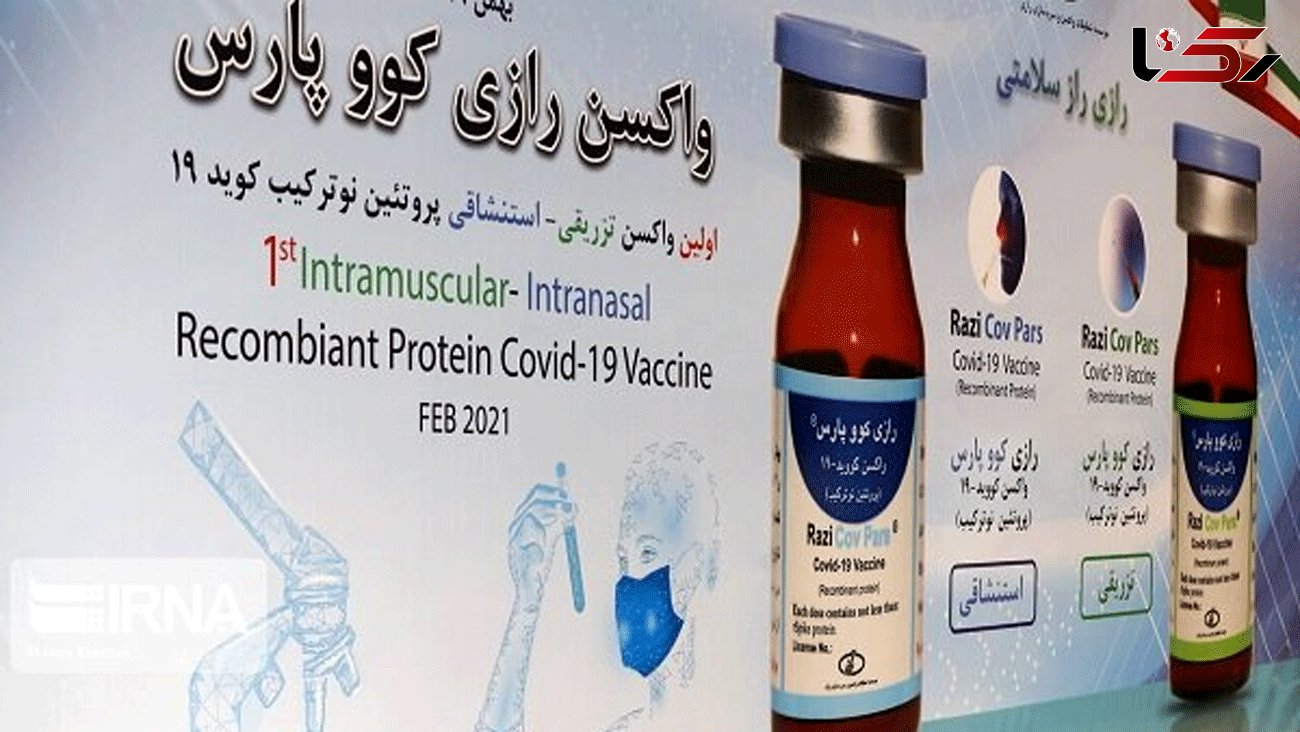
Dr. Jalil Koohpayehzadeh said on Thursday that the first and second phases of the human trial of Razi Cov Pars Covid-19 Vaccine will take about four months. He added that this new Iranian Covid-19 vaccine must accomplish its third phase in order to be verified scientifically.
Iran unveiled its new homegrown Covo-Pars vaccine on February 7, 2021.
The President of Iran University of Medical Sciences also said that the new homegrown vaccine is a recombinant and inhalable vaccine that will be produced using advanced technology, adding that it has successfully passed its laboratory test and the animal trial phase beyond the researchers' expectations.
He expressed hope that there will be good and positive results in the human trial phases so that it can help the country in the fight against the coronavirus.
Dr. Koohpayehzadeh also pointed out that in the first phase, the vaccine will be tested on 133 people, and in the second phase, as many as 500 people divided into two groups of 250 people will be tested.
He continued that "After successfully passing the previous phases, we will prepare for the third phase of the human trial with a population of several thousand people."
Dr. Kuhpayehzadeh added that "This vaccine has three doses, two of which are injectable and one is inhalable. The first 2 doses are injected on days zero and 21 and nasal inhalation is done on day 51."
He went on to say that a website has been established for the volunteers who want to enroll for the human trial of the vaccine, stressing that the volunteers must be of a certain age group with certain physical conditions and they must gain the permission of the Food and Drug Administration first. Follow the Official Rokna NEWS Telegram Channel For More and fresh NEWS.
MHN

Send Comments